PrEP Guidelines and Identifying Individuals Who Need or Want PrEP
PrEP is a highly effective tool for preventing HIV transmission through sex. Clinical guidelines consistently recommend discussing PrEP with all sexually active individuals and offering PrEP to anyone who requests it.1,2
Assessing Individuals Who Need or Want PrEP
- CDC recommends that PrEP should be discussed with all sexually active adults and adolescents who are HIV-negative1
- Clinicians should offer PrEP to anyone who requests it1,2
Factors that increase the chance of HIV-1 acquisition include2:



Methods of HIV Prevention Through Sex and Their Effectiveness
Treatment as prevention (U=U)3,a

PrEP4

Condoms5-7

aCDC updated best estimates for various HIV prevention strategies when taken as prescribed.
bEstimates of effectiveness are for sexual transmission of HIV only.
cEffectively no risk of sexual HIV transmission in people who achieve and maintain viral load <200 copies/mL.
dEfficacy data for prevention of sexual transmission does not include injection drug use.
Summary of Select Recommendations for PrEP
Please see full guidelines or recommendation statements for additional information on these recommendations.
PrEP is recommended for prevention of sexually acquired HIV1 and is a key strategy towards meeting the goals of the EHE initiative.9 See below for select recommendations for the use of PrEP.
- Any licensed prescriber can prescribe PrEP. PrEP is recommended for people without HIV who may be likely to acquire HIV1
- Prescribe PrEP to anyone who asks for it, even if the individual does not report factors for HIV acquisition through sex1
- Safely shortening the time to initiation, including same-day initiation, may be useful for some individuals such as those who have a substantially high likelihood of acquiring HIV through sex in the time between visits for evaluation and PrEP prescription1
- Grade A: In the US, clinicians should prescribe PrEP using effective ART to those with increased likelihood of HIV acquisition through sex2,e
- Because of the USPSTF Grade A recommendation, private health insurance plans and Medicaid expansion programs should cover PrEP and related ancillary tests and services without cost sharing10; the number of times individuals can restart PrEP should not be restricted11
eSexually active adults and adolescents weighing at least 35 kg (77 lb) who have engaged in anal or vaginal sex in the past 6 months with a partner who has HIV or an STI in the past 6 months, or history of inconsistent or no condom use with a partner whose HIV status is not known; those who engage in transactional sex.2
The CDC, USPSTF, WHO, IAS-USA, ACOG, ACHA, and AAP recommend PrEP for HIV prevention.2,12-17
The CDC, IAS-USA, and WHO endorse same-day PrEP use.15,17,18
Click on each button below for a more detailed summary of CDC recommendations for clinically appropriate rapid PrEP start.
- HIV negative test result on the day of PrEP initiation
- Assessment of renal function
- Assistance for benefits navigation, copayment assistance, medication assistance as appropriate
- Education and counseling on PrEP use and adherence
- Provide rapid follow-up contact for people who test HIV positive or have renal dysfunction
- Ongoing monitoring and follow-up care appointments for PrEP users
- Have clinicians available to prescribe/dispense/administer PrEP medication
- A very recent possible HIV exposure was reported but there are no signs and symptoms of acute HIV, evaluate for nPEP before PrEP
- No easy or consistent means of contact for return appointments
- Active mental health conditions are severe enough to interfere with understanding of PrEP requirements (adherence, follow-up visits)
- Ambivalence about starting PrEP is expressed
- Blood cannot be drawn for laboratory testing
- Signs/symptoms and sexual history indicating possible acute HIV are present
- History of renal disease or associated conditions
- No insurance coverage or means to pay when picking up medication that day
- No confirmed means of contact if a laboratory test indicates a need to discontinue PrEP (eg, HIV-positive test result, renal dysfunction)
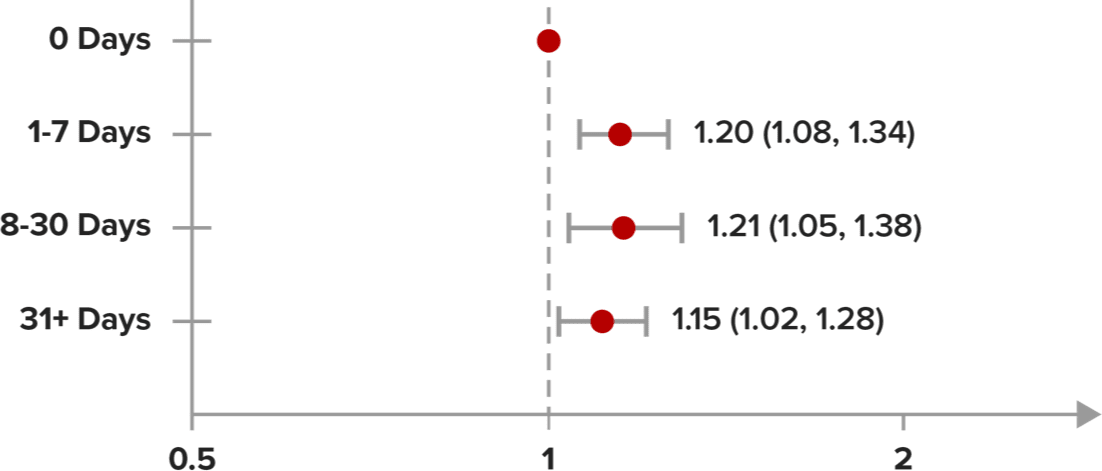
In a claims study, data showed that delayed dispensing of PrEP–regardless of the length of delay–was associated with higher likelihood of acquiring HIV19,f,g
In an open-source data analysis from an IQVIA claims database of people who received a PrEP prescription from January 2019 to February 2023 (N=522,273), it was found that any delay in dispensing of PrEP was associated with an increased chance of acquiring HIV, when compared to same-day initiation of PrEP19
Relationship Between Time to First PrEP Dispensing and Chance of Acquiring HIV Within 12 Months of First PrEP Claim and ≥1 PrEP Claim Dispensed19
Time to first dispense claim |
TotalN |
HIVN |
|---|---|---|
| 0 Days | 80,610 | 1,280 |
| 1-7 Days | 23,774 | 453 |
| 8-30 Days | 13,313 | 254 |
| 31+ Days | 21,606 | 392 |
Odds ratio of Acquiring HIV (95% Cl)


fThis information is an estimate derived from the use of information under license from the following IQVIA information service: IQVIA Longitudinal Access and Adjudication Dataset (IQVIA LAAD), for the period January 2019 through February 2023. IQVIA expressly reserves all rights, including rights of copying, distribution, and republication.
gAmong individuals who have >0 rejected or abandoned claims (including never dispensed).
AAP, American Academy of Pediatrics; ACHA, American College Health Association; ACOG, American College of Obstetricians and Gynecologists; ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; EHE, Ending the HIV Epidemic; IAI, insertive anal intercourse; IAS-USA, International Antiviral Society–USA; MSM, men who have sex with men; nPEP, nonoccupational post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; RAI, receptive anal intercourse; STI, sexually transmitted infection; U=U, undetectable=untransmittable; USPSTF, U.S. Preventive Services Task Force; WHO, World Health Organization.
References:
- Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update: a clinical practice guideline. Published December 2021. Accessed September 29, 2025. https://stacks.cdc.gov/view/cdc/112360
- US Preventive Services Task Force. Preexposure prophylaxis to prevent acquisition of HIV: US Preventive Services Task Force recommendation statement. JAMA. 2023;330(8):736-745. doi:10.1001/jama.2023.14461
- Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171-181. doi:10.1001/jama.2016.5148
- Centers for Disease Control and Prevention. Clinical guidance for PrEP. Updated February 10, 2025. Accessed September 8, 2025. https://www.cdc.gov/hivnexus/hcp/prep/index.html
- Johnson WD, O'Leary A, Flores SA. Per-partner condom effectiveness against HIV for men who have sex with men. AIDS. 2018;32(11):1499-1505. doi:10.1097/QAD.0000000000001832
- Smith DK, Herbst JH, Zhang X, Rose CE. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2015;68(3):337-344. doi:10.1097/QAI.0000000000000461
- Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002;(1):CD003255. doi:10.1002/14651858.CD003255
- Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States-2021 update: a clinical practice guideline. Published December 2021. Accessed September 8, 2025. https://stacks.cdc.gov/view/cdc/112360
- U.S. Department of Health and Human Services. Key EHE strategies. Updated July 15, 2024. Accessed September 8, 2025. https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/key-strategies
- Centers for Medicare and Medicaid Services. FAQs about Affordable Care Act and women’s health and cancer rights act implementation Part 68. Published October 21, 2024. Accessed September 8, 2025. https://www.cms.gov/files/document/faqs-implementation-part-68.pdf
- U.S. Department of Labor. FAQs about Affordable Care Act implementation (Part 47). Published July 19, 2021. Accessed September 8, 2025. https://www.dol.gov/sites/dolgov/files/EBSA/about-ebsa/our-activities/resource-center/faqs/aca-part-47.pdf
- American College of Obstetricians and Gynecologists. Preexposure prophylaxis for the prevention of human immunodeficiency virus. Published June 2022. Accessed September 18, 2025. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/06/preexposure-prophylaxis-for-the-prevention-of-human-immunodeficiency-virus
- American College Health Association. ACHA guidelines. HIV pre-exposure prophylaxis. Published January 2019. Accessed September 18, 2025. https://www.acha.org/wp-content/uploads/2024/06/ACHA_HIV_PrEP_Guidelines_Jan2019.pdf
- Kimball AA, Zhu W, Leonard J, et al. HIV preexposure prophylaxis provision among adolescents: 2018 to 2021. Pediatrics. 2023;152(5):e2023062599. doi:10.1542/peds.2023-062599
- Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA Panel. JAMA. 329(1):63-84. doi:10.1001/jama.2022.22246
- World Health Organization. Pre-exposure prophylaxis (PrEP). Accessed September 18, 2025. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/prevention/pre-exposure-prophylaxis
- Centers for Disease Control and Prevention. PrEP for the prevention of HIV infection in the U.S.: 2021 guideline summary. Published July 2023. Accessed September 18, 2025. https://www.cdc.gov/hivnexus/media/pdfs/2024/04/cdc-hiv-together-brochure-prepguidelineupdate2021-provider.pdf
- World Health Organization. Differentiated and simplified pre-exposure prophylaxis for HIV prevention: update to WHO implementation guidance. Updated July 27, 2022. Accessed September 18, 2025. https://www.who.int/publications/i/item/9789240053694
- Tao L, Yang J, Zachry W, et al. The real-world impact of Pre-exposure Prophylaxis (PrEP) prescription uptake and dispensing status on HIV infection risk in the US. Poster presented at: Infectious Diseases Week; October 11-15, 2023; Boston, MA.